Starting with data
Overview
Teaching: XX min
Exercises: XX minQuestions
First data analysis in R
Objectives
Describe what a data frame is.
Load external data from a .csv file into a data frame.
Summarize the contents of a data frame.
Describe what a factor is.
Convert between strings and factors.
Reorder and rename factors.
Format dates.
Export and save data.
Presentation of the gene expression data
We are going to use part of the data published by Blackmore et al. (2017), The effect of upper-respiratory infection on transcriptomic changes in the CNS. The goal of the study was to determine the effect of an upper-respiratory infection on changes in RNA transcription occuring in the cerebellum and spinal cord post infection. Gender matched eight week old C57BL/6 mice were inoculated with saline or with Influenza A by intranasal route and transcriptomic changes in the cerebellum and spinal cord tissues were evaluated by RNA-seq at days 0 (non-infected), 4 and 8.
The dataset is stored as a comma separated value (CSV) file. Each row holds information for a single RNA expression measurement, and the columns represent:
| Column | Description |
|---|---|
| gene | The name of the gene that was measured |
| sample | The name of the sample the gene expression was measured in |
| expression | The value of the gene expression |
| organism | The organism/species - here all data stem from mice |
| age | The age of the mouse (all mice were 8 weeks here) |
| sex | The sex of the mouse |
| infection | The infection state of the mouse, i.e. infected with Influenza A or not infected. |
| strain | The Influenza A strain; C57BL/6 in all cases. |
| time | The duration of the infection (in days). |
| tissue | The tissue that was used for the gene expression experiment, i.e. cerebellum or spinal cord. |
| mouse | The mouse unique identifier. |
We have already downloaded the data and it can be found in the directory
course-data/data/GSE96870/.
You are now ready to load the data:
rna <- read.csv("course-data/data/GSE96870/rnaseq.csv")
This statement doesn’t produce any output because, as you might recall, assignments don’t display anything. If we want to check that our data has been loaded, we can see the contents of the data frame by typing its name
rna
Wow… that was a lot of output. At least it means the data loaded
properly. Let’s check the top (the first 6 lines) of this data frame
using the function head():
head(rna)
gene sample expression organism age sex infection strain time
1 Asl GSM2545336 1170 Mus musculus 8 Female InfluenzaA C57BL/6 8
2 Apod GSM2545336 36194 Mus musculus 8 Female InfluenzaA C57BL/6 8
3 Cyp2d22 GSM2545336 4060 Mus musculus 8 Female InfluenzaA C57BL/6 8
4 Klk6 GSM2545336 287 Mus musculus 8 Female InfluenzaA C57BL/6 8
5 Fcrls GSM2545336 85 Mus musculus 8 Female InfluenzaA C57BL/6 8
6 Slc2a4 GSM2545336 782 Mus musculus 8 Female InfluenzaA C57BL/6 8
tissue mouse ENTREZID
1 Cerebellum 14 109900
2 Cerebellum 14 11815
3 Cerebellum 14 56448
4 Cerebellum 14 19144
5 Cerebellum 14 80891
6 Cerebellum 14 20528
product
1 argininosuccinate lyase, transcript variant X1
2 apolipoprotein D, transcript variant 3
3 cytochrome P450, family 2, subfamily d, polypeptide 22, transcript variant 2
4 kallikrein related-peptidase 6, transcript variant 2
5 Fc receptor-like S, scavenger receptor, transcript variant X1
6 solute carrier family 2 (facilitated glucose transporter), member 4
ensembl_gene_id external_synonym chromosome_name gene_biotype
1 ENSMUSG00000025533 2510006M18Rik 5 protein_coding
2 ENSMUSG00000022548 <NA> 16 protein_coding
3 ENSMUSG00000061740 2D22 15 protein_coding
4 ENSMUSG00000050063 Bssp 7 protein_coding
5 ENSMUSG00000015852 2810439C17Rik 3 protein_coding
6 ENSMUSG00000018566 Glut-4 11 protein_coding
phenotype_description
1 abnormal circulating amino acid level
2 abnormal lipid homeostasis
3 abnormal skin morphology
4 abnormal cytokine level
5 decreased CD8-positive alpha-beta T cell number
6 abnormal circulating glucose level
hsapiens_homolog_associated_gene_name
1 ASL
2 APOD
3 CYP2D6
4 KLK6
5 FCRL2
6 SLC2A4
## Try also
## View(rna)
Note
read.csv() assumes that fields are delineated by commas, however, in
several countries, the comma is used as a decimal separator and the
semicolon (;) is used as a field delineator. If you want to read in
this type of files in R, you can use the read.csv2() function. It
behaves exactly like read.csv() but uses different parameters for
the decimal and the field separators. If you are working with another
format, they can be both specified by the user. Check out the help for
read.csv() by typing ?read.csv to learn more. There is also the
read.delim() function for reading tab separated data files. It is important to
note that all of these functions are actually wrapper functions for
the main read.table() function with different arguments. As such,
the data above could have also been loaded by using read.table()
with the separation argument as ,. The code is as follows:
rna <- read.table(file = "course-data/data/GSE96870/rnaseq.csv",
sep = ",",
header = TRUE)
The header argument has to be set to TRUE to be able to read the
headers as by default read.table() has the header argument set to
FALSE.
What are data frames?
Data frames are the de facto data structure for most tabular data, and what we use for statistics and plotting.
A data frame can be created by hand, but most commonly they are
generated by the functions read.csv() or read.table(); in other
words, when importing spreadsheets from your hard drive (or the web).
A data frame is the representation of data in the format of a table where the columns are vectors that all have the same length. Because columns are vectors, each column must contain a single type of data (e.g., characters, integers, factors). For example, here is a figure depicting a data frame comprising a numeric, a character, and a logical vector.
We can see this when inspecting the structure of a data frame
with the function str():
str(rna)
'data.frame': 32428 obs. of 19 variables:
$ gene : chr "Asl" "Apod" "Cyp2d22" "Klk6" ...
$ sample : chr "GSM2545336" "GSM2545336" "GSM2545336" "GSM2545336" ...
$ expression : int 1170 36194 4060 287 85 782 1619 288 43217 1071 ...
$ organism : chr "Mus musculus" "Mus musculus" "Mus musculus" "Mus musculus" ...
$ age : int 8 8 8 8 8 8 8 8 8 8 ...
$ sex : chr "Female" "Female" "Female" "Female" ...
$ infection : chr "InfluenzaA" "InfluenzaA" "InfluenzaA" "InfluenzaA" ...
$ strain : chr "C57BL/6" "C57BL/6" "C57BL/6" "C57BL/6" ...
$ time : int 8 8 8 8 8 8 8 8 8 8 ...
$ tissue : chr "Cerebellum" "Cerebellum" "Cerebellum" "Cerebellum" ...
$ mouse : int 14 14 14 14 14 14 14 14 14 14 ...
$ ENTREZID : int 109900 11815 56448 19144 80891 20528 97827 118454 18823 14696 ...
$ product : chr "argininosuccinate lyase, transcript variant X1" "apolipoprotein D, transcript variant 3" "cytochrome P450, family 2, subfamily d, polypeptide 22, transcript variant 2" "kallikrein related-peptidase 6, transcript variant 2" ...
$ ensembl_gene_id : chr "ENSMUSG00000025533" "ENSMUSG00000022548" "ENSMUSG00000061740" "ENSMUSG00000050063" ...
$ external_synonym : chr "2510006M18Rik" NA "2D22" "Bssp" ...
$ chromosome_name : chr "5" "16" "15" "7" ...
$ gene_biotype : chr "protein_coding" "protein_coding" "protein_coding" "protein_coding" ...
$ phenotype_description : chr "abnormal circulating amino acid level" "abnormal lipid homeostasis" "abnormal skin morphology" "abnormal cytokine level" ...
$ hsapiens_homolog_associated_gene_name: chr "ASL" "APOD" "CYP2D6" "KLK6" ...
Inspecting data.frame Objects
We already saw how the functions head() and str() can be useful to
check the content and the structure of a data frame. Here is a
non-exhaustive list of functions to get a sense of the
content/structure of the data. Let’s try them out!
Size:
dim(rna)- returns a vector with the number of rows in the first element, and the number of columns as the second element (the dimensions of the object)nrow(rna)- returns the number of rowsncol(rna)- returns the number of columns
Content:
head(rna)- shows the first 6 rowstail(rna)- shows the last 6 rows
Names:
names(rna)- returns the column names (synonym ofcolnames()fordata.frameobjects)rownames(rna)- returns the row names
Summary:
str(rna)- structure of the object and information about the class, length and content of each columnsummary(rna)- summary statistics for each column
Note: most of these functions are “generic”, they can be used on other types of
objects besides data.frame.
Challenge:
Based on the output of
str(rna), can you answer the following questions?
- What is the class of the object
rna?- How many rows and how many columns are in this object?
- How many genes (as defined by the
genevariable) have been measured in this experiment?Solution
- class: data frame
- how many rows: 32428, how many columns: 19
- how many genes: 1477
Indexing and subsetting data frames
Our rna data frame has rows and columns (it has 2 dimensions), if we
want to extract some specific data from it, we need to specify the
“coordinates” we want from it. Row numbers come first, followed by
column numbers. However, note that different ways of specifying these
coordinates lead to results with different classes.
# first element in the first column of the data frame (as a vector)
rna[1, 1]
# first element in the 6th column (as a vector)
rna[1, 6]
# first column of the data frame (as a vector)
rna[, 1]
# first column of the data frame (as a data.frame)
rna[1]
# first three elements in the 7th column (as a vector)
rna[1:3, 7]
# the 3rd row of the data frame (as a data.frame)
rna[3, ]
# equivalent to head_rna <- head(rna)
head_rna <- rna[1:6, ]
head_rna
: is a special function that creates numeric vectors of integers in
increasing or decreasing order, test 1:10 and 10:1 for
instance. See section \@ref(sec:genvec) for details.
You can also exclude certain indices of a data frame using the “-” sign:
rna[, -1] ## The whole data frame, except the first column
rna[-c(7:32428), ] ## Equivalent to head(rna)
Data frames can be subset by calling indices (as shown previously), but also by calling their column names directly:
rna["gene"] # Result is a data.frame
rna[, "gene"] # Result is a vector
rna[["gene"]] # Result is a vector
rna$gene # Result is a vector
In RStudio, you can use the autocompletion feature to get the full and correct names of the columns.
Challenge
Create a
data.frame(rna_200) containing only the data in row 200 of thernadataset.Notice how
nrow()gave you the number of rows in adata.frame?
Use that number to pull out just that last row in the inital
rnadata frame.Compare that with what you see as the last row using
tail()to make sure it’s meeting expectations.Pull out that last row using
nrow()instead of the row number.Create a new data frame (
rna_last) from that last row.
Use
nrow()to extract the row that is in the middle of thernadataframe. Store the content of this row in an object namedrna_middle.Combine
nrow()with the-notation above to reproduce the behavior ofhead(rna), keeping just the first through 6th rows of the rna dataset.Solution
## 1. rna_200 <- rna[200, ] ## 2. ## Saving `n_rows` to improve readability and reduce duplication n_rows <- nrow(rna) rna_last <- rna[n_rows, ] ## 3. rna_middle <- rna[n_rows / 2, ] ## 4. rna_head <- rna[-(7:n_rows), ]
Factors
Factors represent categorical data. They are stored as integers associated with labels and they can be ordered or unordered. While factors look (and often behave) like character vectors, they are actually treated as integer vectors by R. So you need to be very careful when treating them as strings.
Once created, factors can only contain a pre-defined set of values, known as levels. By default, R always sorts levels in alphabetical order. For instance, if you have a factor with 2 levels:
sex <- factor(c("male", "female", "female", "male", "female"))
R will assign 1 to the level "female" and 2 to the level
"male" (because f comes before m, even though the first element
in this vector is "male"). You can see this by using the function
levels() and you can find the number of levels using nlevels():
levels(sex)
[1] "female" "male"
nlevels(sex)
[1] 2
Sometimes, the order of the factors does not matter, other times you
might want to specify the order because it is meaningful (e.g., “low”,
“medium”, “high”), it improves your visualization, or it is required
by a particular type of analysis. Here, one way to reorder our levels
in the sex vector would be:
sex ## current order
[1] male female female male female
Levels: female male
sex <- factor(sex, levels = c("male", "female"))
sex ## after re-ordering
[1] male female female male female
Levels: male female
In R’s memory, these factors are represented by integers (1, 2, 3),
but are more informative than integers because factors are self
describing: "female", "male" is more descriptive than 1,
2. Which one is “male”? You wouldn’t be able to tell just from the
integer data. Factors, on the other hand, have this information built
in. It is particularly helpful when there are many levels (like the
species names in our example dataset).
Converting to factors {-}
If you need to convert a factor to a character vector, you use
as.character(x).
as.character(sex)
[1] "male" "female" "female" "male" "female"
Renaming factors {-}
When your data is stored as a factor, you can use the plot()
function to get a quick glance at the number of observations
represented by each factor level. Let’s look at the number of males
and females in our data.
plot(sex)
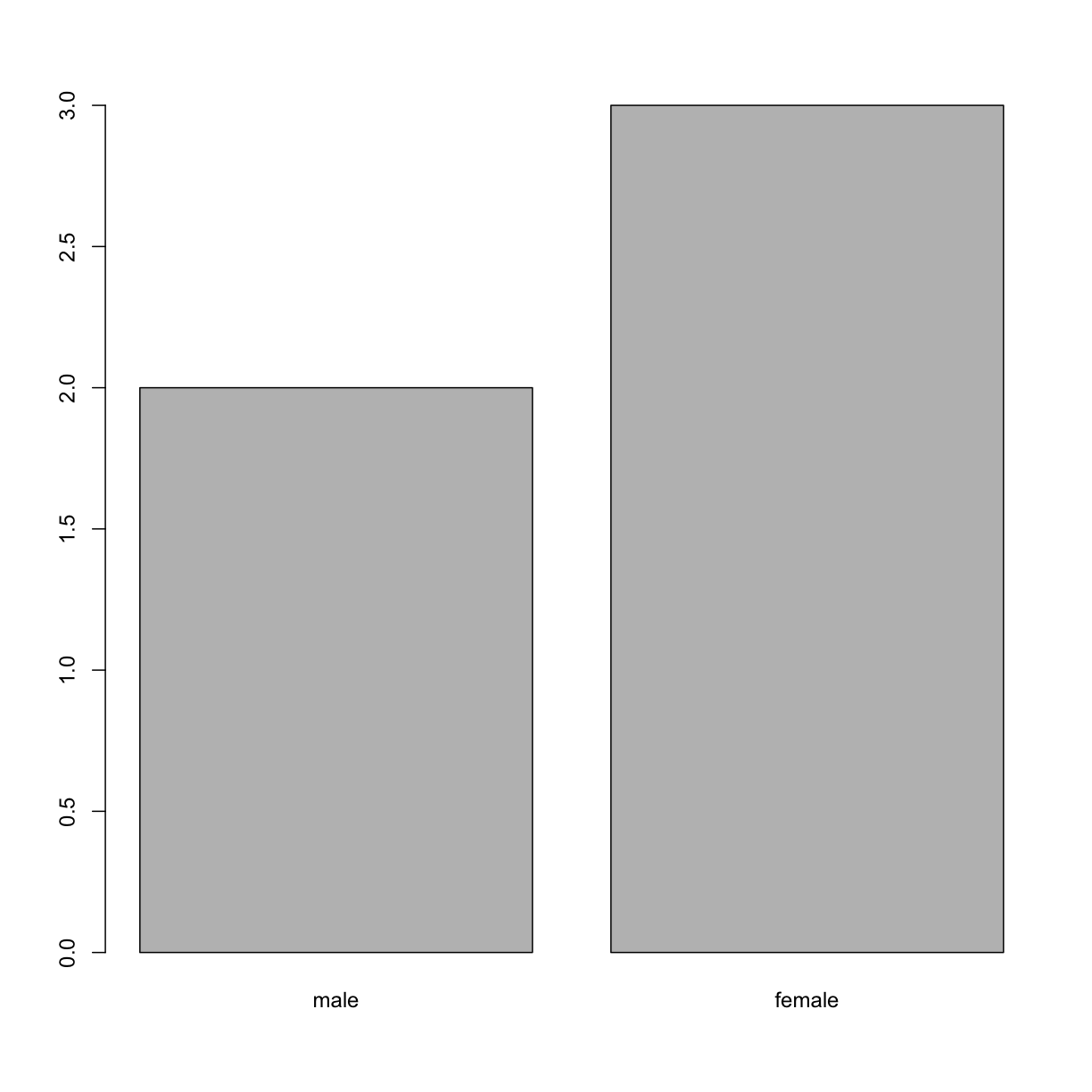
If we want to rename these factor, it is sufficient to change its levels:
levels(sex)
[1] "male" "female"
levels(sex) <- c("M", "F")
sex
[1] M F F M F
Levels: M F
plot(sex)
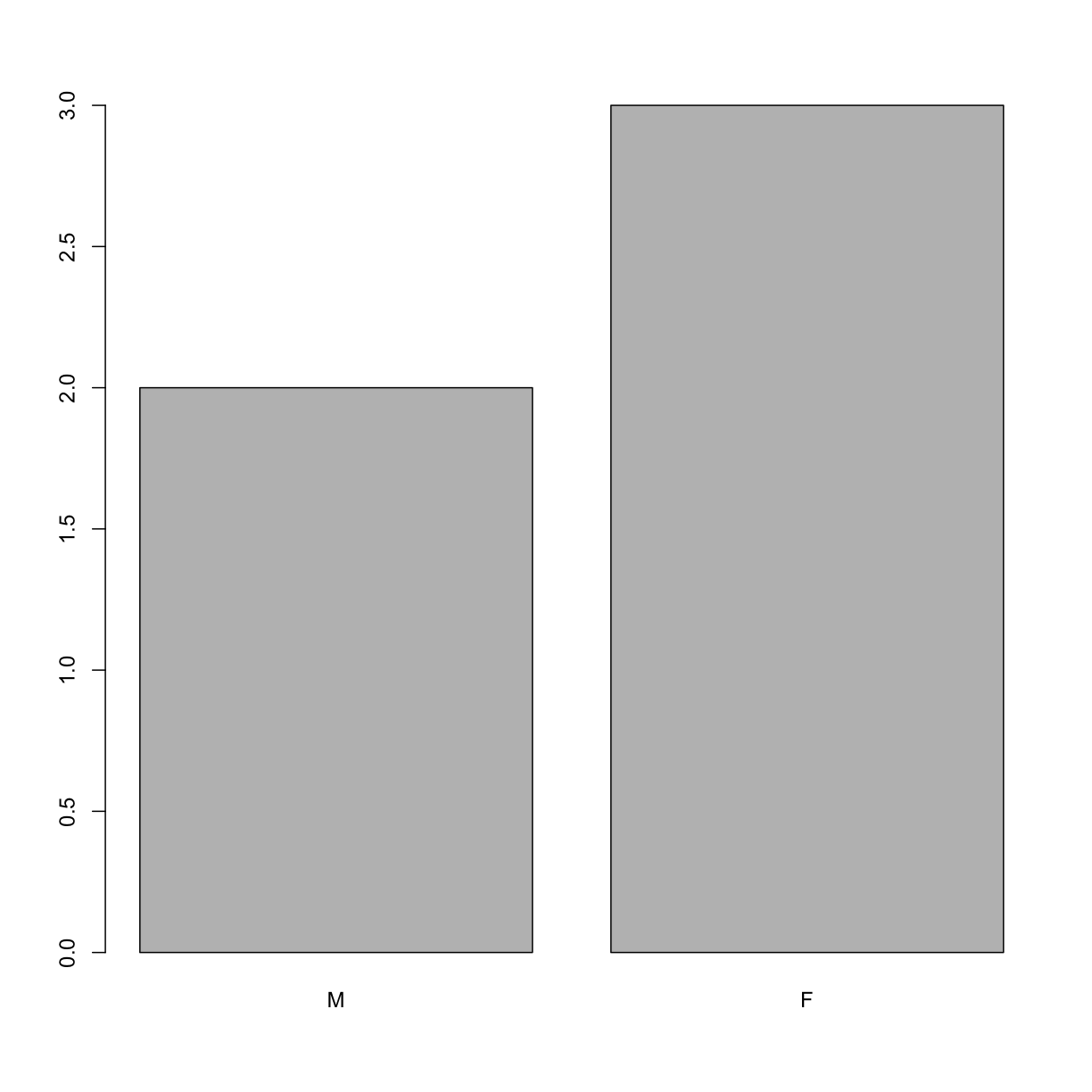
Homework Challenge:
- Rename “female” and “male” to “Female” and “Male” respectively.
Challenge:
We have seen how data frames are created when using
read.csv(), but they can also be created by hand with thedata.frame()function. There are a few mistakes in this hand-crafteddata.frame. Can you spot and fix them? Don’t hesitate to experiment!animal_data <- data.frame( animal = c(dog, cat, sea cucumber, sea urchin), feel = c("furry", "squishy", "spiny"), weight = c(45, 8 1.1, 0.8))Solution
- missing quotations around the names of the animals
- missing one entry in the “feel” column (probably for one of the furry animals)
- missing one comma in the weight column
Homework Challenge:
Can you predict the class for each of the columns in the following example?
Check your guesses using
str(country_climate):
Are they what you expected? Why? Why not?
Try again by adding
stringsAsFactors = TRUEafter the last variable when creating the data frame? What is happening now?stringsAsFactorscan also be set when reading text-based spreadsheets into R usingread.csv().country_climate <- data.frame( country = c("Canada", "Panama", "South Africa", "Australia"), climate = c("cold", "hot", "temperate", "hot/temperate"), temperature = c(10, 30, 18, "15"), northern_hemisphere = c(TRUE, TRUE, FALSE, "FALSE"), has_kangaroo = c(FALSE, FALSE, FALSE, 1) )
The automatic conversion of data type is sometimes a blessing, sometimes an annoyance. Be aware that it exists, learn the rules, and double check that data you import in R are of the correct type within your data frame. If not, use it to your advantage to detect mistakes that might have been introduced during data entry (a letter in a column that should only contain numbers for instance).
Learn more in this RStudio tutorial
Matrices
Before proceeding, now that we have learnt about dataframes, let’s
recap package installation and learn about a new data type, namely the
matrix. Like a data.frame, a matrix has two dimensions, rows and
columns. But the major difference is that all cells in a matrix must
be of the same type: numeric, character, logical, … In that
respect, matrices are closer to a vector than a data.frame.
The default constructor for a matrix is matrix. It takes a vector of
values to populate the matrix and the number of row and/or
columns1. The values are sorted along the columns, as illustrated
below.
m <- matrix(1:9, ncol = 3, nrow = 3)
m
[,1] [,2] [,3]
[1,] 1 4 7
[2,] 2 5 8
[3,] 3 6 9
Homework Challenge:
Using the function
installed.packages(), create acharactermatrix containing the information about all packages currently installed on your computer. Explore it.Solution:
## create the matrix ip <- installed.packages() head(ip) ## try also View(ip) ## number of package nrow(ip) ## names of all installed packages rownames(ip) ## type of information we have about each package colnames(ip)
It is often useful to create large random data matrices as test
data. The exercise below asks you to create such a matrix with random
data drawn from a normal distribution of mean 0 and standard deviation
1, which can be done with the rnorm() function.
Homework Challenge:
Construct a matrix of dimension 1000 by 3 of normally distributed data (mean 0, standard deviation 1)
Solution
set.seed(123) m <- matrix(rnorm(3000), ncol = 3) dim(m)[1] 1000 3head(m)[,1] [,2] [,3] [1,] -0.56047565 -0.99579872 -0.5116037 [2,] -0.23017749 -1.03995504 0.2369379 [3,] 1.55870831 -0.01798024 -0.5415892 [4,] 0.07050839 -0.13217513 1.2192276 [5,] 0.12928774 -2.54934277 0.1741359 [6,] 1.71506499 1.04057346 -0.6152683
Summary of R objects
So far, we have seen several types of R object varying in the number of dimensions and whether they could store a single of multiple data types:
vector: one dimension (they have a length), single type of data.matrix: two dimensions, single type of data.data.frame: two dimensions, one type per column.
Lists
A data type that we haven’t seen yet, but that is useful to know, and follows from the summary that we have just seen are lists:
list: one dimension, every item can be of a different data type.
Below, let’s create a list containing a vector of numbers, characters, a matrix, a dataframe and another list:
l <- list(1:10, ## numeric
letters, ## character
installed.packages(), ## a matrix
cars, ## a data.frame
list(1, 2, 3)) ## a list
length(l)
[1] 5
str(l)
List of 5
$ : int [1:10] 1 2 3 4 5 6 7 8 9 10
$ : chr [1:26] "a" "b" "c" "d" ...
$ : chr [1:399, 1:16] "abind" "annotate" "AnnotationDbi" "AnnotationHub" ...
..- attr(*, "dimnames")=List of 2
.. ..$ : chr [1:399] "abind" "annotate" "AnnotationDbi" "AnnotationHub" ...
.. ..$ : chr [1:16] "Package" "LibPath" "Version" "Priority" ...
$ :'data.frame': 50 obs. of 2 variables:
..$ speed: num [1:50] 4 4 7 7 8 9 10 10 10 11 ...
..$ dist : num [1:50] 2 10 4 22 16 10 18 26 34 17 ...
$ :List of 3
..$ : num 1
..$ : num 2
..$ : num 3
List subsetting is done using [] to subset a new sub-list or [[]]
to extract a single element of that list (using indices or names, of
the list is named).
l[[1]] ## first element
[1] 1 2 3 4 5 6 7 8 9 10
l[1:2] ## a list of length 2
[[1]]
[1] 1 2 3 4 5 6 7 8 9 10
[[2]]
[1] "a" "b" "c" "d" "e" "f" "g" "h" "i" "j" "k" "l" "m" "n" "o" "p" "q" "r" "s"
[20] "t" "u" "v" "w" "x" "y" "z"
l[1] ## a list of length 1
[[1]]
[1] 1 2 3 4 5 6 7 8 9 10
Exporting and saving data
Exporting tabular data {-}
We have seen how to read a text-based spreadsheet into R using the
read.table family of functions. To export a data.frame to a
text-based spreadsheet, we can use the write.table set of functions
(write.csv, write.delim, …). They all take the variable to be
exported and the file to be exported to. For example, to export the
rna data to an rna.csv file in the data_output
directory, we would execute:
write.csv(rna, file = "data_output/rna.csv")
This new csv file can now be shared with other collaborators who aren’t familiar with R.
Saving data {-}
Exporting data to a spreadsheet has several limitations, such as those
described in the first chapter such as possible inconsistencies with
, and . for decimal separators and lack of variable type
definitions. Furthermore, exporting data to a spreadsheet is only
relevant for rectangular data such as dataframes and matrices.
A more general way to save data, that is specific to R and is
guaranteed to work on any operating system, is to use the save
function. Saving objects will generate a binary representation of the
object on disk, a R Data file (rda extension) that guarantees to
produce the same object once loaded back into R using the load
function.
save(rna, file = "data_output/rna.rda")
rm(rna)
load("data_output/rna.rda")
head(rna)
Note about how the function load loads the object in the file
directly in the global environment.
There is also the saveRDS and readRDS functions that save R
objects to binary files (using the rds extension here) and read
these back into R. From a user’s perspective, main difference is that,
load loads an object in the global environment while readRDS reads
the data from disk and returns it. It is thus necessary to store the
output of readRDS in a variable:
saveRDS(rna, file = "data_output/rna.rds")
rm(rna)
rna <- readRDS("data_output/rna.rds")
head(rna)
To conclude, when it comes to saving data from R that will be loaded again in R, saving and loading is the preferred approach. If tabular data need to be shared with somebody that is not using R, then exporting to a text-based spreadsheet is a good alternative.
-
Either the number of rows or columns are enough, as the other one can be deduced from the length of the values. Try out what happens if the values and number of rows/columns don’t add up. ↩
Key Points
Tabular data in R